While discussing states of matter, i casually asked the kids when we drop a solid in liquid, does the solid float or sink? Together they told that the any solid sinks in liquid.
I dropped an ice block (a solid) in water ( a liquid) they were surprised to see it float. Even a block of wood or plastic floats on water. Whereas when I dropped a piece of metal like nail, it sank to the bottom.

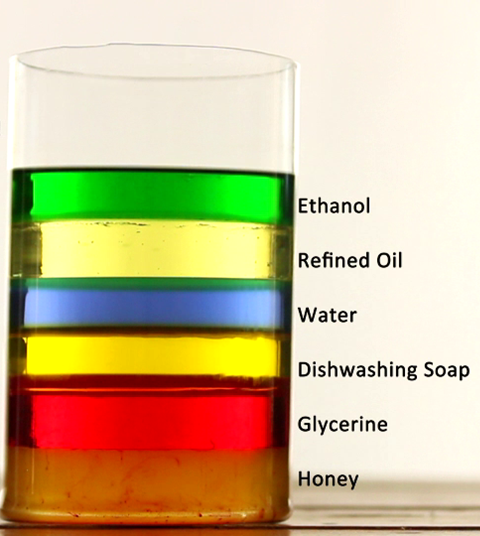
Curiosity took us to next level. Kids were interested to know what happens when we mix two liquids? We did a simple experiment of mixing water and oil.
We took a two small glass jars. To the first, we added water followed by oil. They clearly formed two separate layers with oil on top.
But kids weren’t convinced. They thought oil is sticky so it’s denser and has to stay at the bottom. So the second jar, I added oil first followed by water. Even then oil stayed on the top. Kids mixed them hard. They were successful in making an emulsion ( mixture of water and oil) which eventually seperated to form two layers.

To clear the confusion, introduced them to the concept of Density. Density is the amount of matter contained in a unit volume of a material.
Mass is the amount of matter contained and volume is the space occupied by a material. So density is also defines as mass by volume.
We took equal quantities of water and oil and weighed them to find that oil weighs less than water. So oil is lighter than water and floats in the top of water. Hence we concluded that denser objects/fluids sink and the lighter ones float.
Similarly, ice,wood and plastic float on water as they are less dense than water whereas a piece of nail as it is more dense than water.
The session ended with an exciting lava lamp experiment. In a big jar we poured water and oil. Then we added a drop of water based food colour (red) to it. It was a spectacular scene to see the red drop sink through the oil layer without mixing in it. Once it reached the water surface, it bursted in the water to dissolve in it.

Then we dropped few Alka seltzer tablets into the jar. The tablets dissolved in water to release bubbles of carbon-di-oxide. Gas bubbles being lighter than oil rose to the top carrying a red coloured water drops along with it. When the bubbles reached the surface the broke and the red colour water drop fell to the bottom through the oil layer.

The whole process resembles a lava and hence the name Lava Lamp.
Very interesting Saipriya!
LikeLike